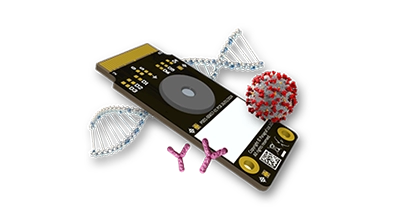
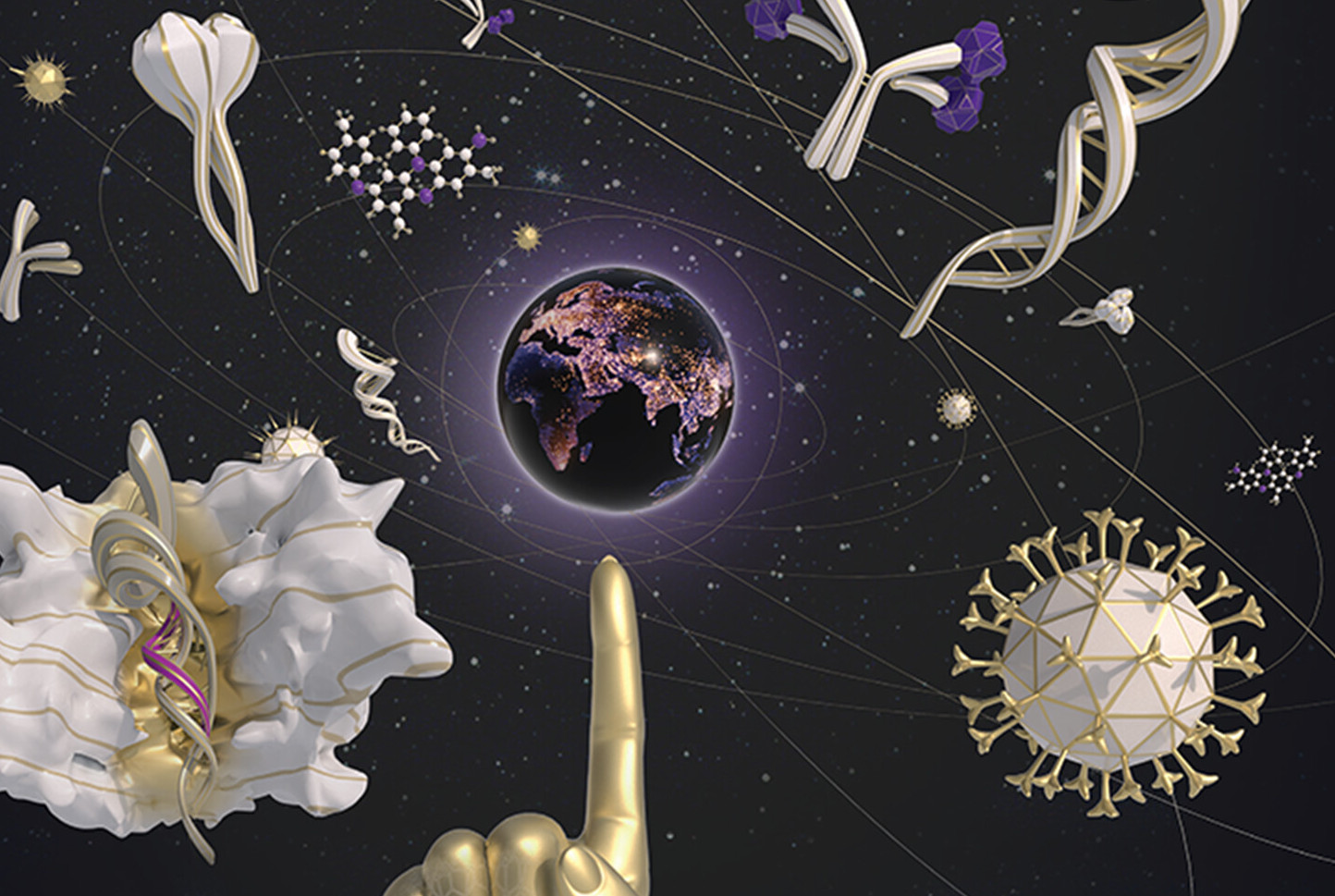
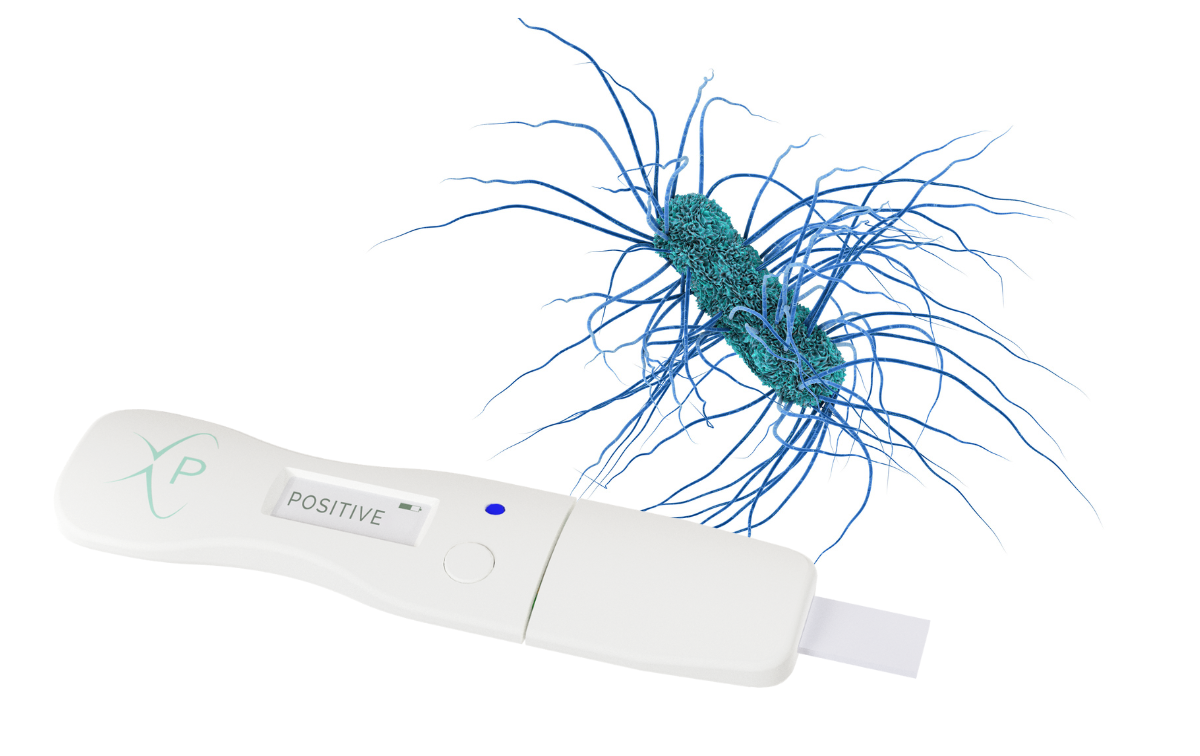
Graphene biosensors enable sensitive, real-time measurement of biomolecules directly from samples without enrichment or amplification, reducing time to result and cost without sacrificing accuracy. Paragraf biosensors have the potential to enable the accuracy of a laboratory test with the ease-of-use of a lateral flow instrument, all the way from handheld point-of-care devices to high throughput screening applications.
Our BPU™ Platform is exclusively available through either the Innovation or Commercial Partnerships. If you’re interested in becoming a partner, you can apply below.
Our commercial partners are using the capabilities of the BPU Platform that have already been demonstrated, utilized and in some cases even published on whereas the innovation partners are engaged in proof-of-concept and pilot projects to test and demonstrate entirely new applications and use cases on the platform.
Applications
Life Science Research
Graphene-based biosensors can be used to measure in real time the dynamic interactions of proteins, small molecules and nucleic acids. Applications include:
- Measuring binding kinetics of proteins, peptides, small molecules and even RNA for drug discovery research
- Optimisation of the development of CRISPR gene editing tools for future plant, animal and human applications

Precision Agriculture
Paragraf graphene biosensors can be used to address the increasing demand for precision in agriculture by improving the quality of both the plants and animals we cultivate, and the environments in which we do so. Their sensitivity, robustness and delivery of real-time data make them ideally placed for use in both laboratory and field settings.
For example:
- CRISPR based biosensing can be deployed in agricultural research laboratories, and even at field sites, to improve plant and animal breeding programmes and to monitor presence of GMOs throughout the supply chain.
- Real-time, distributed protein or nucleic acid biosensing can be applied to detect the presence of infectious diseases in plants or animals in the field, enabling more rapid detection and intervention to prevent spread.
- Multiomics biosensing (simultaneous protein and nucleic acid detection) could be deployed to field sites to monitor water and soil health, feeding back real time information to farmers allowing them to target their inputs precisely and reduce over use of water, fertiliser and pesticides.
Healthcare
Paragraf’s graphene based biosensors are currently for research use only but have potential in the future to be developed into powerful tools with which to tackle major challenges in healthcare. Our core biosensor platform’s capabilities to deliver sensitive, real time and low cost measurements in either high throughput laboratory or single use ‘point of need’ formats are ideally suited to use in healthcare.
Future applications could include:
- Pathogen and drug resistance detection
- Metabolic and cardiovascular disease detection and monitoring
- Recurrence monitoring in cancer

Scientific papers
Related news
Apply to partner with us!
Our growing network of partners operate in markets across diagnostics, research, agriculture, defense and more – all leveraging Paragraf’s BPU Platform to build unrivalled products that create and disrupt their markets.
To apply for partnership, let us know a little bit about you, your team, the idea you want to commercialize with us and what will make you our next great partner.